Research: Photonic Biocomputing
Optical Computing in Living Cells
The newest project in our lab is creating systems for in vivo photonic computation. Physical computational approaches have recently emerged as an attractive alternative to conventional computational platforms to solve large combinatorial optimization problems. This advantage stems from the fact that most optimization problems can be mapped onto spin Hamiltonians. Solving the optimization problems entails finding the ground state of the spin system. Various physical platforms have been used to demonstrate this idea with varying degrees of success. While exciting, these systems all suffer from significant disadvantages — specifically the need to operate at liquid helium temperatures and the necessity to perform difficult, expensive and environmentally damaging nanofabrication processes to create them.
We are working to create microorganisms containing in vivo fluorescent protein coacervates capable of performing physical computation at room temperature when self-assembled with other such microorganisms in biofilms. This platform promises a revolution in biological computing: polariton computation operates on a timescale more than twelve orders of magnitude faster than optogenetics. In order to build this vision, we need to create a toolkit of biological parts capable of performing each function separately and then combine them in a single organism. The first step was our recent demonstration of room-temperature polariton condensation and thermalization in the red fluorescent protein mScarlett.
We are collaborating on this project with Vinod Menon at CCNY Physics, Allie Obermeyer at Columbia Chemical Engineering, and Giovanna Ghirlanda at Arizona State Chemistry
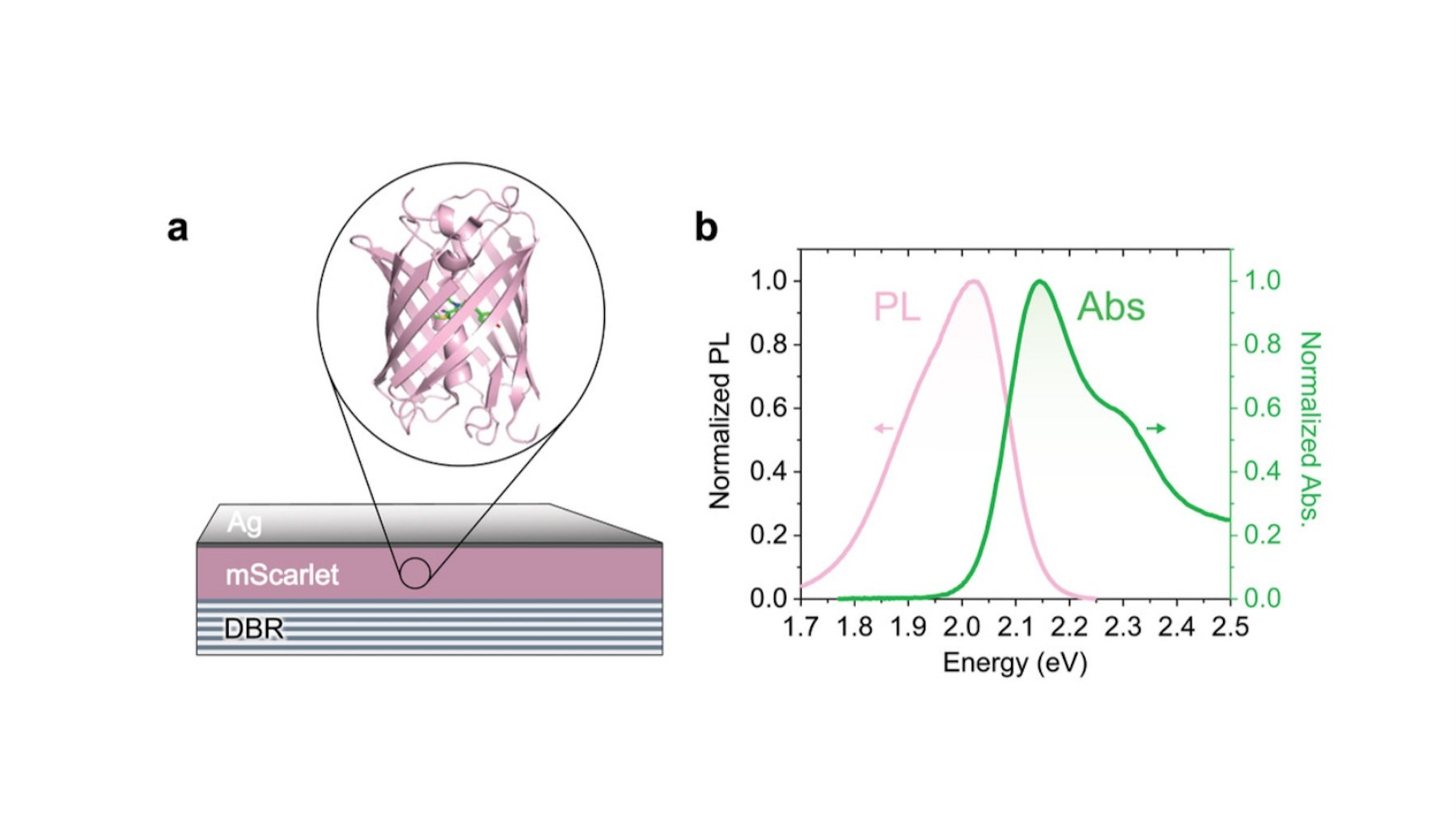
(Left) Schematic of the fluorescent protein mScarlett in a microcavity formed by a lower distributed Bragg reflector and an upper silver mirror layer. (Right) Absorption and Emission spectra of mScarlett on a quartz substrate.
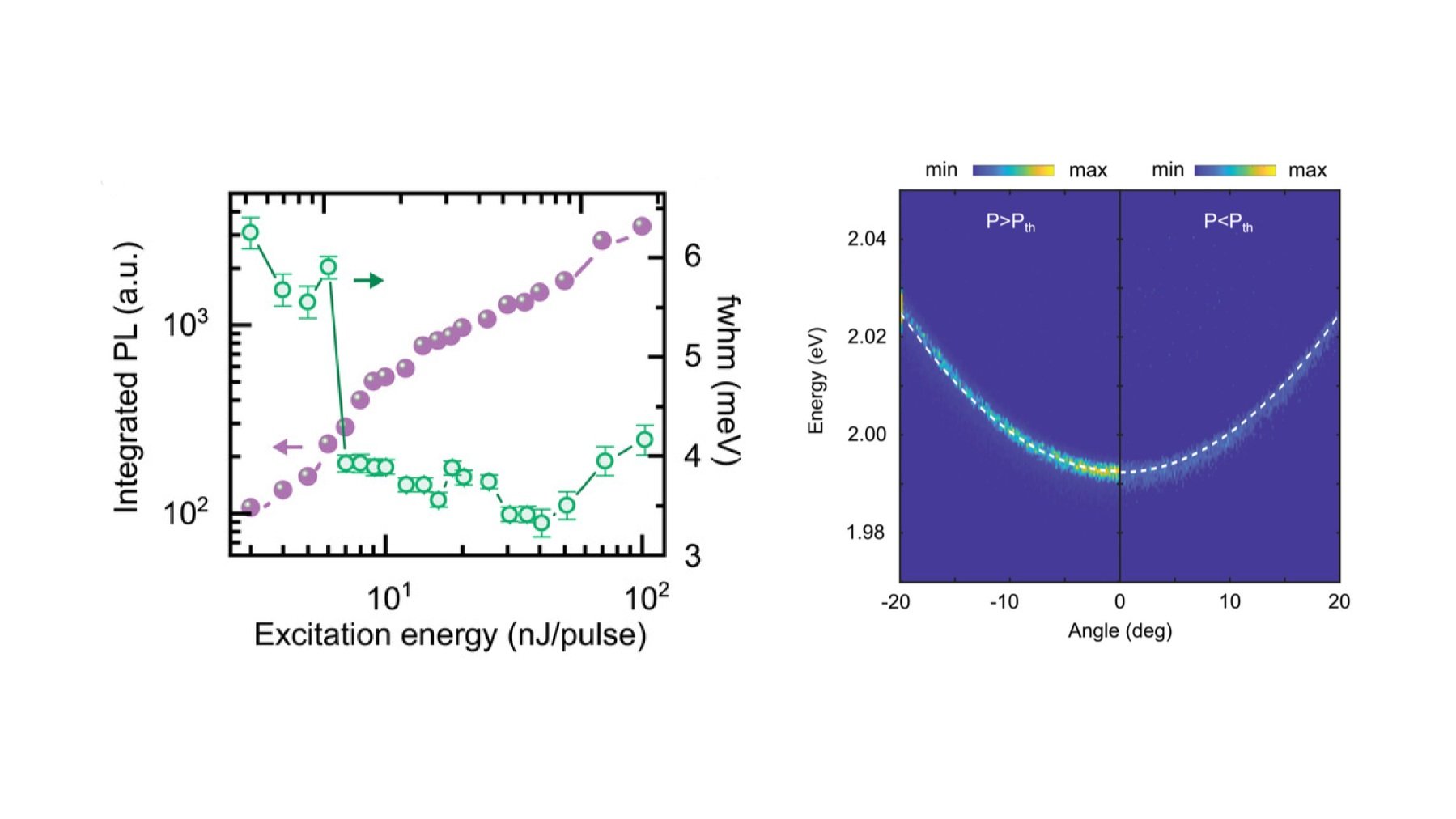
(Left) Integrated PL intensity along with linewidth as a function of different excitation powers for the microcavity. (Right) Angle-resolved fluorescence maps of the microcavity below and above the thermalization threshold (15.6 mJ cm−2).